FeS
Due to motivations based on cosmic abundance, models of Earth formation,
and ability to dissolve into liquid iron, sulphur seems to be one of the
most likely light elements in the core.
It has been suggested(1) that the presence of dissolved sulphur would
increase the viscosity
of liquid iron, because sulphur atoms would aggregate to form chains,
impeding the diffusion of the atoms.
We have used first principles techniques to study the structural, dynamical,
and electronic structure properties of a liquid alloy of iron and sulphur
(12 % mole concentration) under Earth's core condition.
We did not observe any formation of aggregate of sulphur, rather, a
repulsion between sulphurs is evident.
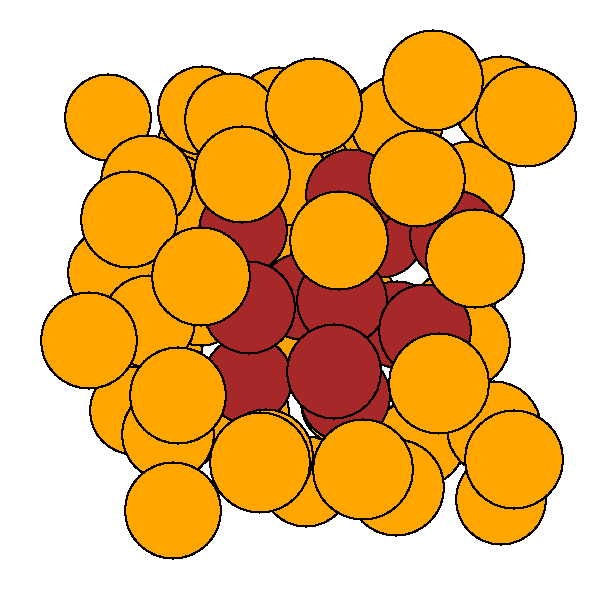
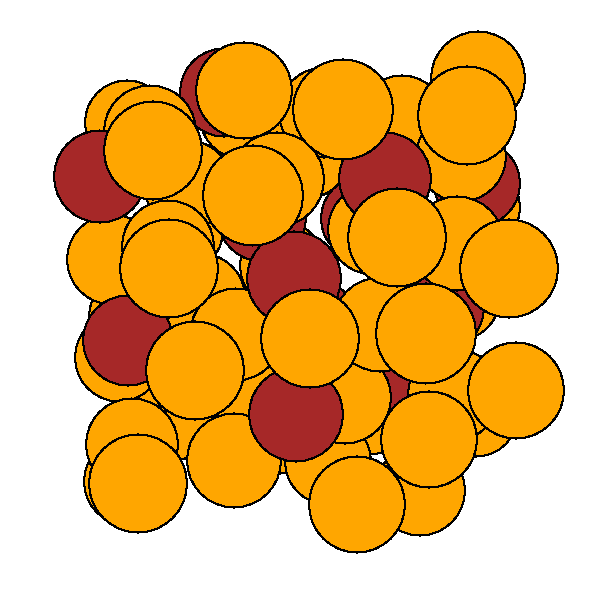 We
gave sulphur all the possible chances to aggregate by starting one of our
first principles molecular dynamics simulation with a cluster of sulphurs
(left image). After only 1 ps the cluster was completely dissociated, and
the sulphur atoms randomly distributed throughout the cell (right image).
We
gave sulphur all the possible chances to aggregate by starting one of our
first principles molecular dynamics simulation with a cluster of sulphurs
(left image). After only 1 ps the cluster was completely dissociated, and
the sulphur atoms randomly distributed throughout the cell (right image).
The dinamical properties of the liquid are similar to those of pure
iron, in particular the viscosity
is the same.
(1) G. E. LeBlanc and R. A. Secco, Geophys. Res. Lett.,
23, 213 (1996).
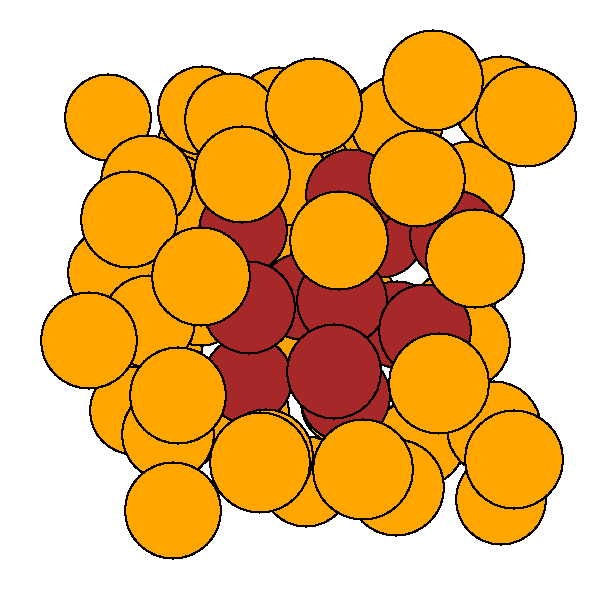
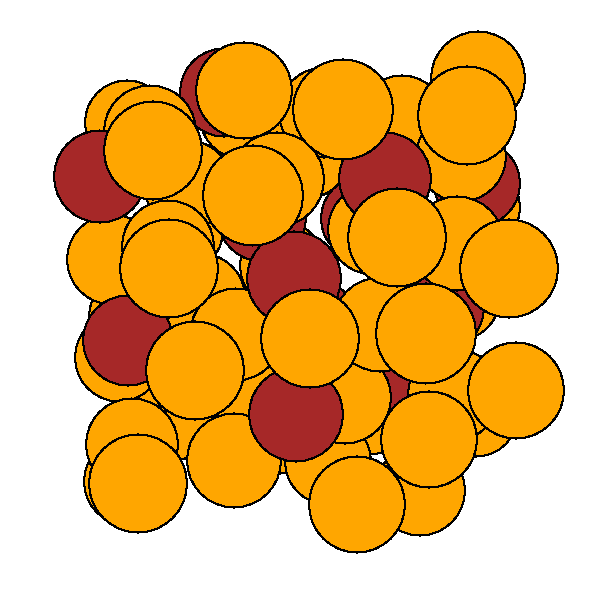 We
gave sulphur all the possible chances to aggregate by starting one of our
first principles molecular dynamics simulation with a cluster of sulphurs
(left image). After only 1 ps the cluster was completely dissociated, and
the sulphur atoms randomly distributed throughout the cell (right image).
We
gave sulphur all the possible chances to aggregate by starting one of our
first principles molecular dynamics simulation with a cluster of sulphurs
(left image). After only 1 ps the cluster was completely dissociated, and
the sulphur atoms randomly distributed throughout the cell (right image).